Which Statement Describes a Reaction at Equilibrium
In the atmosphere under the effect of ultraviolet rays oxygen molecules combine to form ozone molecules and ozone. II and III only D.
4 the same and the reaction continues in both directions.
. Question 30 1 point Which statement best describes general equilibrium. I II and III. At equilibrium the total concentration of products equals the.
At dynamic equilibrium the rate of the forward reaction is higher than the rate of the reverse reaction. Aug 2006-19 A chemical reaction is at equilibrium. Which statement describes a chemical reaction at equilibrium.
3 the same and the reaction has stopped. 4 The reaction mixture is not complete as reactants are still being added to the system. The concentrations of the products and reactants are equal.
Le Chateliers Principle If a system is at equilibrium and any change is made to concentration pressure or temperature the reaction will shift to undo the effect of the stress and a new equilibrium is re-established. At dynamic equilibrium the concentrations of reactants and products are equal. 3 the reactions in baking are not reversible.
They consist mainly of reactant molecules. S of reactants and products do not change. I and II only B.
Which statement correctly describes a reaction in dynamic equilibrium. There is only one set of equilibrium concentrations that equals the Ke value. Which statement describes the molecules and a reaction once it reaches equilibrium.
Equilibrium is reached when the reaction stops. Which statements are correct for a reaction at equilibrium. 3 The concentrations of the products and reactants are equal.
Compared to the rate of the forward reaction the rate of the reverse reaction is. Chemistry questions and answers. The concentrations of the products and reactants are constant.
Compared to the rate of the forward reaction the rate of the reverse reaction is. That is the rate of forward reaction is equal to the rate of backward reaction. At dynamic equilibrium the reactions stop and the amount.
Equilibrium is reached when the reaction stops. 1 The products are completely consumed in the reaction. The forward and reverse reactions both continue.
Faster and more reactant is produced. 4 The concentrations of the products and reactants are constant. Which statement describes this reaction at equilibrium.
At equilibrium the rate of the forward reaction is the same as the rate of the reverse reaction. 1 The rate of the forward and backward reactions are equal. Thus we can conclude that out of the given options statement the concentrations of the products and reactants are equal describes a chemical reaction at equilibrium.
At dynamic equilibrium the reactions continue but the amounts of reactants and products do not change. 2 The reactants are completely consumed in the reaction. The concentrations of reactants and products are equal.
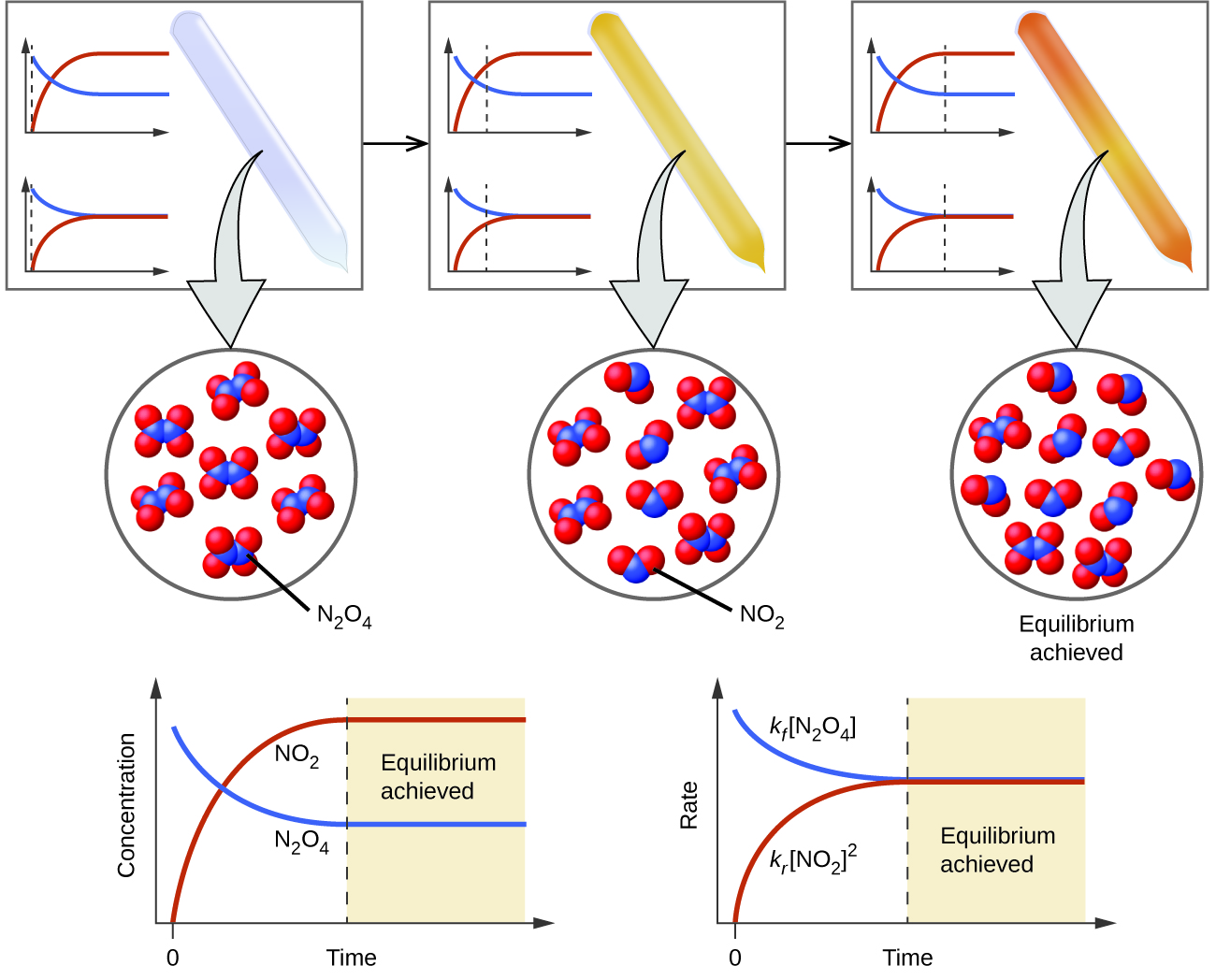
2 faster and more product is produced. 1 the concentration of N2O4 g must equal the concentration of NO2 2 The concentration of N2O4 g and the concentration of NO2must be constant 3 The rate of the forward reaction is. There is only one set of equilibrium concentrations that equals the Kc value At equilibrium the rate of the forward reaction is the same as the rate of the reverse reaction At equilibrium the total concentration.
They maintain stable concentrations. A chemical reaction is at equilibrium. 1 faster and more reactant is produced.
I and III only C. 2No changes will be apparent as the forward and reverse continue. QUESTION 33 Which statement best describes general equilibrium.
The rates of the forward and reverse reactions are equal. At dynamic equilibrium the reactions stop but the amounts of. At dynamic equilibrium the forward and reverse reactions stop.

Welcome To Learnapchemistry Com Ap Chemistry Question Paper Ap Chem

Comments
Post a Comment